Abstract
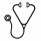
Introduction: Patients with RR-AML, particularly older adults, have dismal outcomes and limited therapy options. Given their tolerability, HMAs are often used to treat RR-AML patients. Whether their mutational profile will affect outcomes in RR-AML patients treated with HMAs is not known. Additionally, a 10-day course of decitabine has been shown to result in higher response rates in AML patients with unfavorable risk cytogenetic abnormalities compared to patients with intermediate- or favorable-risk cytogenetic in the upfront setting. However, little is known about the impact of different treatment schedules in in patients with RR-AML. In a prior multicenter study, we showed that HMAs are a reasonable option for patients with RR-AML. Using the same multicenter international database, we analyzed whether specific mutations and different therapeutic schedules of HMA are able to predict response and survival in this population.
Methods: Data was collected, spanning a period from 2006 to 2016, from 7 centers in the United States and 4 centers in Europe of patients treated with HMAs for RR-AML. Responses were defined by modified International Working Group 2003 criteria. Kaplan-Meier methods estimated overall survival (OS) from initiation of HMAs to death or end of follow-up. Multivariable logistic regression models estimated odds for response, and multivariable Cox Proportional Hazard (CPH) models estimated hazards ratios (HR) for OS. Molecular data for FLT3, NPM1 and TP53 and the administration schedules of decitabine (5- or 10-days) and azacitidine (7 consecutive days, 5 consecutive days, and 7-day schedule with a weekend break [5-2-2 schedule]) were the independent variables of interest in this analysis. Missing data were imputed using the Multivariate Imputation by Chained Equation approach implemented with the mice package in R with 10 iterations per variable. All tests were two-sided, with an alpha significance level of 0.05. All analyses were performed using R version 3.3.2.
Results: Of 655 patients, 365 (56%) had relapsed and 290 (44%) had refractory AML; only 2% had good risk karyotype, while 40% had poor risk karyotype based on the MRC cytogenetic risk classification for AML. About 43.8% of patients received the approved 7-day schedule of azacitidine, while 10.2% and 3% of patients received a 5-day schedule and the 5-2-2 schedule, respectively. Decitabine was given on a 5-day and 10-day schedule in 30.8% and 8.5% of patients, respectively. In a multivariate logistic regression analysis, variables that were significantly associated with higher odds of achieving CR/CRi included presence of 5% PB blasts (OR = 2.05, 95%CI, 1.25-3.37, p-value = 0.0046), a duration of CR1 of longer than 6 months (OR = 1.76, 95% CI 1.14-2.71, p=0.0108), and a 10-day schedule of Decitabine (OR =2.23, 95% CI 1.13-4.43, p-value= 0.0215) [Figure 1A]. In a multivariate CPH model for OS, the percentage of circulating blasts (PB blasts >5% vs. ≤ 5%, HR 1.38, 95%CI, 1.11-1.71, p= 0.0041), blasts in the BM (BM blasts >20% vs. ≤ 20%, HR 1.23, 95%CI, 1.01-1.49, p= 0.00405) and a platelet count of ≤ 30 vs. > 30 x109/L (HR 1.24, 95% 1.01-1.52, p=0.0401) were significantly associated with inferior OS [Figure 1B]. Importantly, neither agenor adverse risk karyotype (including complex and monosomal karyotype) were associated with worse response rates and OS. Patients who received the 10-day schedule of decitabine had a higher CR/CRi rate than patients who received other HMA schedules (28% vs. 15.7%, p=0.04). However, their OS was not significantly different (8 months vs. 6.6 months, p=0.13) [Figure 2]. Data regarding FLT3, NPM1 and TP53 mutational status were available in 269, 228 and 64 patients, of whom 17%, 24% and 6% were found to have FLT3, NPM1 and TP53 mutations, respectively. Patients with NPM1, FLT3 or TP53 mutations did not have worse response rates or OS compared to patients without these mutations [Figure 3 and Figure 4].
Conclusions: In one of the largest reported cohorts of patients with RR-AML treated with HMAs, we found that NPM1, FLT3 and TP53 mutational status did not impact response or OS. Consistent with a recent published study in the upfront setting, RR-AML patients who received the 10-day schedule of decitabine had a higher CR/CRi rate than patients who received other HMA schedules; however, this did not translate in improved OS.
Montesinos: Celgene Corporation: Honoraria, Research Funding. Itzykson: Novartis: Research Funding; Janssen: Research Funding. Ritchie: Novartis: Consultancy, Other: Research funding to my institution, and travel, Speakers Bureau; Celgene: Consultancy, Other: Travel, Speakers Bureau; Bristol-Myers Squibb: Other: Research funding to my institution; NS Pharma: Other: Research funding to my institution; Pfizer: Consultancy, Other: Research funding to my institution; Astellas Pharma: Other: Research funding to my institution; Incyte: Consultancy, Speakers Bureau. Sekeres: Celgene: Membership on an entity's Board of Directors or advisory committees. Podoltsev: Alexion: Consultancy; Ariad: Consultancy; Incyte: Consultancy; CTI biopharma/Baxalta: Consultancy. Fathi: Seattle Genetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Pfizer: Honoraria; Medimmune: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Komrokji: Celgene: Honoraria; Novartis: Honoraria, Speakers Bureau. Santini: Celgene: Honoraria, Research Funding; Novartis: Honoraria; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Otsuka: Consultancy; Janssen: Consultancy, Honoraria; Amgen: Membership on an entity's Board of Directors or advisory committees. Brunner: Celgene: Research Funding; Takeda: Research Funding. Roboz: Cellectis: Research Funding; AbbVie, Agios, Amgen, Amphivena, Array Biopharma Inc., Astex, AstraZeneca, Celator, Celgene, Clovis Oncology, CTI BioPharma, Genoptix, Immune Pharmaceuticals, Janssen Pharmaceuticals, Juno, MedImmune, MEI Pharma, Novartis, Onconova, Pfizer, Roche Pharmace: Consultancy. Fenaux: Janssen: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding. Germing: Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Janssen: Honoraria. Zeidan: AbbVie, Otsuka, Pfizer, Gilead, Celgene, Ariad, Incyte: Consultancy, Honoraria; Takeda: Speakers Bureau; Otsuka: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal